
- 31 Jul 2025
- Ryan Mordue
Challenging the Rulebook: Navigating Drug Discovery Beyond the Rule of 5 (BRo5)

As a biologist navigating the ever-evolving landscape of therapeutic modalities – and especially drug discovery Beyond the Rule of 5 – our technical challenges change constantly. Yet, our ultimate goal remains unchanged: to develop potent, efficacious molecules that deliver the desired therapeutic effect (without off-target activity), while being well-absorbed, appropriately distributed, and efficiently cleared from the body.
Traditionally, medicinal chemists have relied on a set of guidelines known as the ‘Rule of 5’ (Ro5) to help identify drug-like molecules with favourable pharmacokinetic properties. These rules were designed to reduce attrition and cost by filtering out compounds unlikely to succeed due to poor absorption or permeability. The Ro5 was introduced by Pfizer chemist Christopher Lipinski (Lipinski et al., 1997). His analysis of the physicochemical properties of orally active drugs led to four key criteria, showing that poor absorption or permeation is more likely when there are more than:
- 5 hydrogen bond donors
- 10 hydrogen bond acceptors
- A molecular weight over 500
- A calculated Log P greater than 5
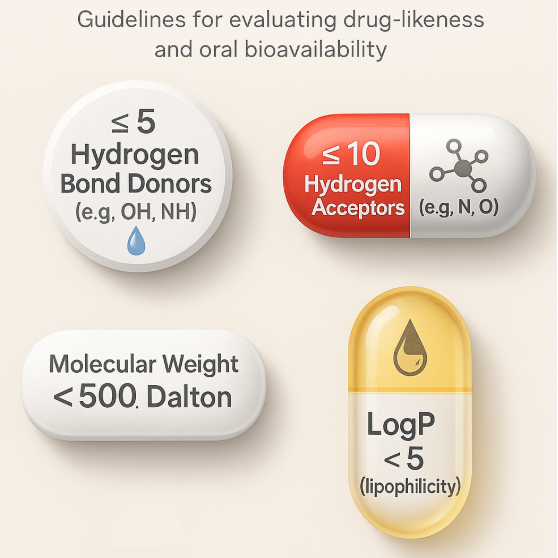
Each rule includes a multiple of five; the Ro5 quickly became the guideline which has helped shape the development of many successful drugs, including ibuprofen, which was discovered in Nottingham by chemists at Boots (which – fun fact – is at the site where I’m writing this article!) With a molecular weight of 206, a Log P just under 4, two hydrogen bond donors, and one acceptor, ibuprofen satisfies all Ro5 criteria – a textbook example of a successful Ro5-compliant drug.
The Ro5 was never intended as a rigid framework; it was intended as a design aid, not a gatekeeper. Strict adherence to the Ro5 has, at times, limited exploration into novel chemical spaces, potentially leaving behind promising therapeutic candidates. It is important to remember the context in which the Ro5 emerged. It was introduced during an era dominated by high-throughput screening (HTS), where discovery programs cast wide nets – screening hundreds of thousands, sometimes millions, of compounds in search of viable leads. In such resource-intensive environments, applying filters like the Ro5 made practical sense. If a compound had more favourable properties and a lower risk of attrition, why wouldn’t we prioritise it? Who said we can’t screen the other less favourable compounds if we fail to find a candidate for development?
However, as time has progressed, HTS, once a cornerstone of early discovery, became less prevalent as the shift to more strategic, tailoring of libraries occurred to identify molecules that could target a specific binding site. Consider kinase ATP-binding pockets (a classic example) where focused libraries of tens of thousands of compounds replaced the millions once screened. This refinement has gone even further with the rise of fragment-based libraries, often containing just approximately 500 to 3,000 compounds. These libraries prioritise pharmacophoric potential over sheer volume, marking a significant departure from past practices.
In essence, the landscape of drug discovery has evolved dramatically. Not just in how we screen compounds, but in the very nature of the therapeutic targets we pursue. Since the introduction of Lipinski’s Ro5, we have seen what some might call a scientific rebellion against its constraints. However, I view this shift not as defiance, but as a natural evolution driven by the expanding toolkit at our disposal.
While traditional tools still hold value, the rise of in-silico screening and AI-driven platforms is reshaping our approach. These technologies not only reduce our reliance on physical compound libraries, but also enable us to explore less defined, more complex binding interfaces. As a result, we are witnessing a true revolution in drug development, one where precision, computational power, and creativity converge to unlock new therapeutic possibilities.
It is with this revolution, we are witnessing the re-emergence of ‘beyond Rule of 5’ (bRo5) molecules, including peptides, oligonucleotides, PROTACs and other TACs, large macrocycles, and other complex modalities that defy traditional boundaries, yet show immense therapeutic promise. Many of these molecules have been a large part of the therapeutic toolbox for decades, but it is the resurgence of these modalities (along with the newcomers) combined with all the new tools that we have available that makes me excited for the new generation of therapeutics.
We now know that clinical success does not always require strict compliance to the Rule of 5. For example, semaglutide (Rybelsus®) and MK-0616, two peptides, have oral bioavailability of just 1–2%, yet both demonstrate remarkable clinical efficacy (Buckley et al., 2018; Johns et al., 2023). These examples underscore a critical point: efficacy and therapeutic value can outweigh strict adherence to physicochemical rules.
So, it is important to remember the Rule of 5 remains a useful compass, but it is no longer the only map. As we continue to innovate, we must balance the wisdom of established guidelines with the courage to explore uncharted territory. After all, the future of medicine may lie not in following the rules – but in knowing when to break them
At our site in Nottingham, we are proud to contribute to the discovery of next generation of therapeutics; pushing the boundaries of what is possible, exploring new modalities such as peptides, oligonucleotides, and a plethora of bivalent molecules that now exist, all while embracing complexity so that it may lead to a better outcome for patients.
So, stay tuned, in the coming weeks and months we will be delving deeper into these modalities to explore their therapeutic potential, and give our opinions on some of the advancements made since the Ro5 was first published.
Welcome to Beyond Rule of 5 at CatSci.
Ryan Mordue, PhD – Bioscience Team Leader.
Explore our Bioscience capabilities here.
Johns, D.G. et al. (2023) ‘Orally Bioavailable Macrocyclic Peptide That Inhibits Binding of PCSK9 to the Low Density Lipoprotein Receptor’, Circulation, 148(2), pp. 144–158. Available at: https://doi.org/10.1161/circulationaha.122.063372.
Lipinski, C.A. et al. (1997) ‘Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings’, Advanced Drug Delivery Reviews, 23(1–3), pp. 3–25. Available at: https://doi.org/10.1016/s0169-409x(96)00423-1.