- 14 May 2025
- Joseph Renny
Complex Synthetic Medicines: Unlocking the Next Frontier in Drug Development
Complex Synthetic Medicines: Unlocking the Next Frontier in Drug Development – Dr Joe Renny
As the pharmaceutical industry continues to innovate at pace, driven by increasingly complex therapeutic challenges and unmet medical needs, the significance of chemistry-led drug development has risen sharply. At the centre of this evolution lies a new generation of chemically engineered therapeutics — complex synthetic medicines. These entities represent the convergence of advanced synthetic chemistry, innovative drug delivery systems, and a profound understanding of biological systems.
This article seeks to define complex synthetic medicines, explore their growing importance within the pharmaceutical landscape, and reflect on the unique scientific, technical, and regulatory challenges associated with their development.
Defining Complex Synthetic Medicines
Complex synthetic medicines are chemically derived therapeutic agents distinguished by their structural intricacy, multi-step synthesis, and often highly specific modes of action. They occupy a distinctive space between conventional small molecules and large biological therapeutics (biologics), combining advantageous properties of both classes while addressing their respective limitations.
Figure 1 provides an overview of some of the key differentiators between traditional small molecules, complex synthetics, and biologics:

Unlike traditional small molecules, which are often limited in their capacity to modulate difficult biological targets, complex synthetic medicines possess the architectural sophistication required to engage challenging biological pathways. At the same time, they overcome several drawbacks of biologics, particularly those related to stability, immunogenicity, and manufacturing constraints.
The defining feature of complex synthetic medicines is not solely their molecular weight or size, but rather the multidimensional complexity of their chemical structure. This complexity often arises from the presence of multiple stereocentres, macrocyclic or dendritic frameworks, and conjugation with targeting ligands or delivery systems such as nanoparticles or liposomes. Their development routinely requires sophisticated synthetic methodologies, precise stereochemical control, and advanced analytical characterisation techniques.
Therapeutic Modalities and Applications
Complex synthetic medicines encompass a diverse array of modalities, each with distinct therapeutic applications. The best way to appreciate their potential is to examine medicines already reshaping standard capacity.
Antibody-Drug Conjugates (ADCs)
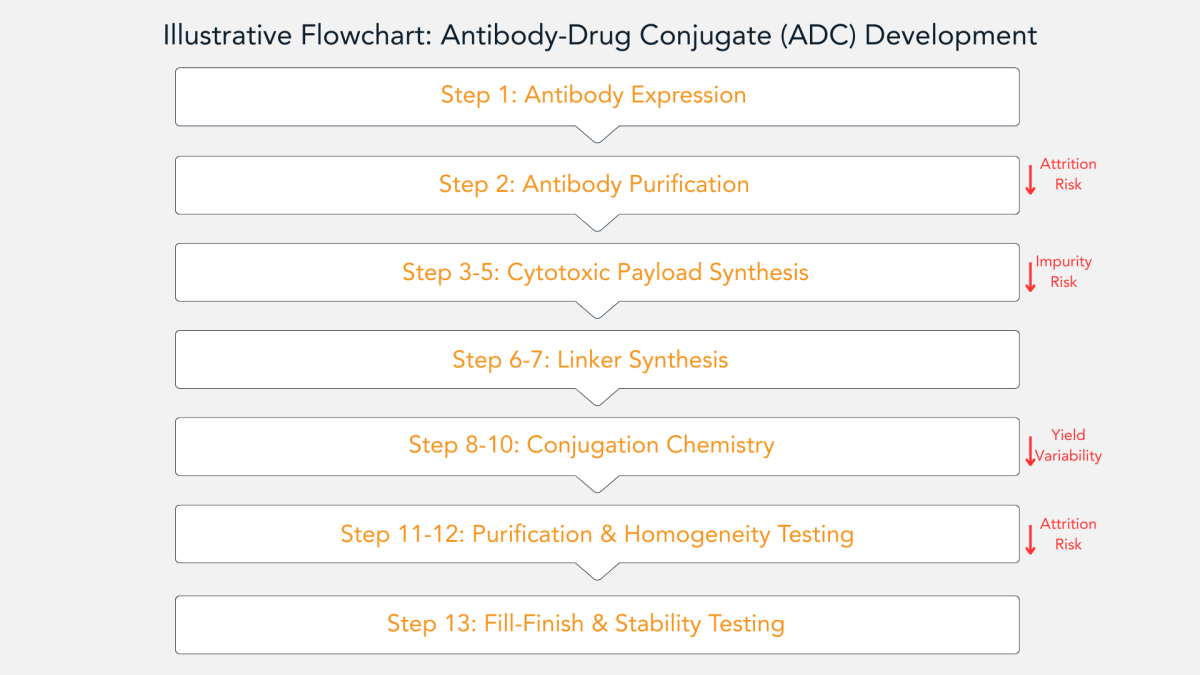
By tethering ultrapotent small molecule payloads to tumour-seeking antibodies, ADCs deliver high potency with selectivity. Figure 2 demonstrates an illustrative overview of the entire development arc, from monoclonal antibody expression and linker engineering through site-specific conjugation, purification, formulation, and sterile fill-finish, spotlighting the key quality and safety inflection points that transform this elegant concept into a reliable, scalable therapy.
Oligonucleotides
Oligonucleotide therapeutics, such as antisense oligonucleotides (ASOs) and small interfering RNAs (siRNAs), represent another prominent class of complex synthetic medicines. These agents exert their pharmacological effects through modulation of gene expression, rendering them particularly valuable in the treatment of rare and genetic disorders.
Peptides and Peptide-Drug Radioconjugates
Peptides, including peptide-drug conjugates, are widely utilised for their capacity to offer high specificity with reduced systemic toxicity. The conjugation of peptides with cytotoxic payloads has significantly enhanced their potential in oncology, where selective tumour targeting is paramount.
Peptide-radioconjugates, particularly those used in neuroendocrine tumours, demonstrate the convergence between synthetic chemistry, tumour targeting, and radioisotope delivery.
Dendrimers
Dendrimers, with their highly branched macromolecular architecture, provide opportunities for multivalent interactions and improved pharmacokinetic profiles. Nanomedicines, utilising carriers such as liposomes or polymeric nanoparticles, facilitate enhanced drug delivery, allowing therapeutic agents to reach sites that would otherwise be inaccessible or prone to rapid degradation.
Collectively, these modalities have enabled the treatment of a growing spectrum of diseases, ranging from oncology and rare genetic disorders to infectious diseases and central nervous system (CNS) conditions. Their capacity to overcome the limitations of traditional therapeutics has positioned complex synthetic medicines at the forefront of modern drug development.
“Complex synthetic medicines are redefining the boundaries of chemical innovation, targeting previously intractable biological pathways with unprecedented precision.”
— Dr Ross Burn, CatSci Ltd
Market Momentum
Commercial data emphasises the speed with which complex modalities are gaining traction amidst their scientific promise. Investor enthusiasm is not speculative; it is grounded in robust sales trajectories. Here, I will explore some of the key players in the complex synthetic space that are driving the growth:
Peptide Radioconjugate
The peptide radioconjugate market, worth an estimated $3.73 billion in 2024, is projected to reach $12.84 billion by the end of 2030 for an impressive 19.21% CAGR. The growth is driven by favourable survival data, expanding theranostic adoption, and global investment in 1777Lu and 225Ac isotope production. Supply chain fragility for medical isotopes and the capital intensity of hot-cell infrastructure pose threats to the growth, but the clinical value proposition is compelling.
The leading drugs in this space are Pluvicto, reaching sales of $1.392 billion in 2024, and Lutathera, achieving $724 million in 2024, delivering cytotoxic βradiation directly to prostate and neuroendocrine tumours, respectively. Furthermore, diagnostic counterparts such as OctreoScan and Illuccix illustrate how diagnostic imaging and therapy are converging.
Leqvio (Inclisiran)
Oligonucleotides reached a new commercial milestone with Legvio (Inclisiran), an siRNA-based therapy for hypercholesterolemia. The drug achieved sales of $754 million in 2024, driven by expanded indications, geographical expansion, and compelling clinical trial data. Its performance confirms that chemically-stabilised siRNA, delivered in a simple sub-cutaneous format, can achieve blockbuster heights, suggesting commercial viability for the wider oligonucleotide field.
However, these complex molecules are not without their challenges. This is an area I will delve into further, but Figure 3 illustrates the synthesis, purification, and lipid nanoparticle (LNP) formulation, along with some associated challenges.

ADCs
Global revenue from ADCs surpassed $5 billion in 2024, spearheaded by Enhertu and Adcetris. Enhertu couples a HER2-targeting antibody to a topoisomerase I inhibitor via a cleavable tetrapeptide linker, achieving global sales of roughly $3.75 billion in 2024 as successive gastric, breast and lung cancer indications expanded its market reach.
Adcetris, meanwhile, generated around $1.911 billion in 2024 by selectively delivering MMAE to CD30-positive lymphomas. It is expected to accelerate further following its first-line diffuse large B-cell lymphoma (DLBCL) label extension. Continued clinical successes in solid tumours and haematological malignancies are widening their addressable patient population, driving sustained double-digit growth.
The figures above demonstrate that complex synthetic medicines are not niche innovations; they are an increasingly central and rapidly growing pillar of the global therapeutics market.
Scientific and Technical Challenges
Despite their considerable promise, the development of complex synthetic medicines presents a range of scientific and operational challenges that far exceed those encountered in the manufacture of conventional small molecules.
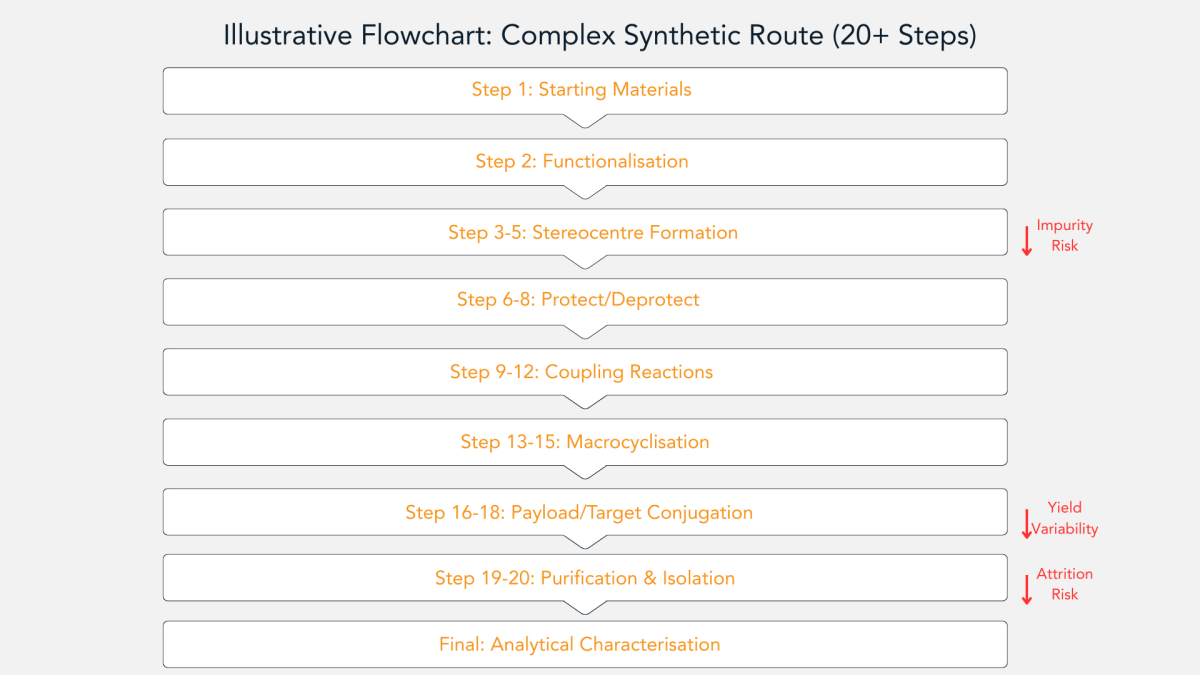
As demonstrated in Figure 4, synthesis often requires upwards of twenty discrete chemical steps, with each transformation demanding rigorous control of reaction conditions, stereochemistry, and impurity profiles. The risk of yield attrition increases exponentially with each additional synthetic step, making route design and process optimisation critical considerations at the earliest stages of development.
Scaling these processes from laboratory to manufacturing scale further complicates development. Many complex synthetic medicines possess properties, such as low solubility, sensitivity to heat or light, and high potency, that necessitate specialised containment facilities and bespoke reactor designs. Ensuring the safety of operators handling potent intermediates, particularly within Occupational Exposure Band (OEB) 4 or 5 environments, introduces additional layers of operational complexity.
Furthermore, analytical characterisation presents its own suite of challenges. The structural intricacy of these molecules often demands a combination of chromatographic, spectroscopic, and bioanalytical methods to fully elucidate purity, potency, and identity. Techniques such as high-performance liquid chromatography-mass spectrometry (HPLC-MS), nuclear magnetic resonance (NMR) spectroscopy, and tailored bioassays become indispensable in ensuring product quality and regulatory compliance.
Regulatory Considerations
Regulatory agencies globally have recognised the unique challenges posed by complex synthetic medicines and are evolving their frameworks accordingly. In the United States, the Food and Drug Administration (FDA) has introduced hybrid approval pathways specifically for complex generics, acknowledging that conventional bioequivalence assessments may be insufficient. Similarly, proposals around the concept of “regulatory density” have emerged, advocating for a risk-adaptive oversight strategy proportionate to the therapeutic profile of the molecule.
In Europe, the European Medicines Agency (EMA) has demonstrated increasing flexibility in its evaluation of advanced therapeutics, particularly where innovative delivery systems or novel mechanisms of action are concerned. Nonetheless, regulatory expectations regarding characterisation, stability, and process validation remain exacting, necessitating a proactive and collaborative approach between drug developers and regulators.
“The synthesis, characterisation, and scalable manufacture of complex synthetic medicines demand a level of technical rigour that challenges even the most experienced development teams.”
— Dr Nigel Richardson, CatSci Ltd
Global Centres of Excellence in Complex Synthetic Medicines
While the development of complex synthetic medicines is a global endeavour, several regions have emerged as leading centres of innovation. The United States, particularly the biotech hubs of Boston and San Diego, continues to drive scientific and commercial leadership, supported by a well-established venture capital ecosystem and regulatory leadership from the FDA.
In the United Kingdom, cities such as Cambridge and Oxford are at the vanguard of academic-industrial collaboration, leveraging world-class research institutions and government-backed initiatives in advanced manufacturing. Europe, more broadly, has embraced personalised medicine as a strategic priority, investing heavily in platforms that integrate chemical and biological modalities.
Asia, with particular growth in China and Japan, has rapidly scaled oligonucleotide and peptide manufacturing capabilities, supported by significant government investment and a burgeoning biotechnology sector. Collectively, these regions are shaping the future trajectory of complex synthetic medicine development.
Risks, Safety, and Developability Considerations
As with any innovative therapeutic class, complex synthetic medicines are not without safety considerations. Their novel structures and mechanisms of action can pose risks of off-target effects, immunogenic responses, or accumulation within specific tissues. Ensuring chiral purity, controlling material impurities, and characterising degradation pathways are essential to mitigate these risks.
Developability assessments — evaluating the feasibility of manufacturing, analytical characterisation, and delivery — play an increasingly critical role in early-stage decision making. For emerging biotech companies, partnering with expert organisations possessing specialised CMC (Chemistry, Manufacturing, and Controls) capabilities can be the determining factor between success and costly programme failure.
The Future of Complex Synthetic Medicines
Looking ahead, the trajectory of complex synthetic medicines is poised for further acceleration. Advances in genomics, proteomics, and computational drug design are enabling the creation of increasingly tailored therapeutics. Innovations in delivery systems, including tissue-specific nanoparticles and biodegradable carriers, are expanding the therapeutic window of potent agents.
Moreover, regulatory frameworks are expected to continue evolving, fostering an environment conducive to innovation while safeguarding patient safety. Strategic collaboration between academia, industry, and regulatory bodies will be pivotal in translating chemical innovation into clinical and commercial success.
At CatSci, we operate at the intersection of these disciplines, collaborating with partners striving to develop molecules that defy conventional limitations.
CatSci is the global leader in complex synthetic medicines development, with expertise across oligonucleotide development, peptide synthesis, translational bioscience, and complex small molecules.
If you would like more information or support developing a complex synthetic medicine, you can get in touch with CatSci for a free initial consultation through the website here.
Useful Resources
- Medicines Discovery Catapult — https://md.catapult.org.uk/
- FDA Guidance on Complex Generics — https://www.fda.gov/drugs/guidance-compliance-regulatory-information/guidances-drugs
- EMA Advanced Therapies — https://www.ema.europa.eu/en/human-regulatory/research-development/advanced-therapies
- State of the Discovery Nation 2021 Report — https://md.catapult.org.uk/resources/state-of-the-discovery-nation-2021/